Connected Classrooms: Engineering
Michael Senra, associate professor of chemical and biomolecular engineering, and his students welcomed a fourth grade class from Paxinosa Elementary as part of the Landis Center for Community Engagement’s Connected Classrooms initiative.
Concepts covered: Chemical engineering, physical changes and food science, DNA, acid rain
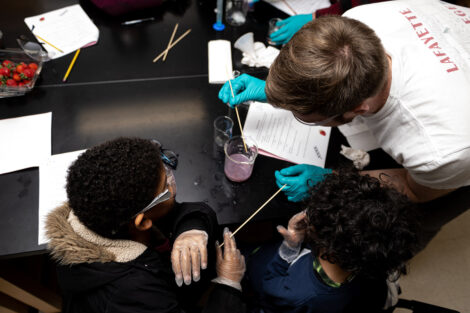
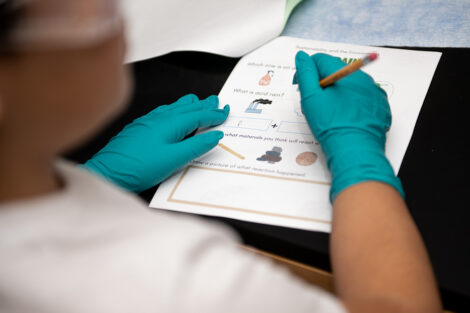
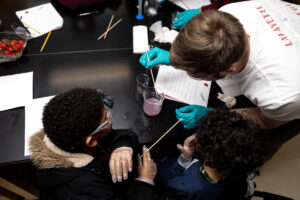
Activities: Making “elephant toothpaste” from hydrogen peroxide and yeast, freezing ice cream in a bag, handout on sustainability and the environment, students dropping vinegar (“acid rain”) on structures to see how they’re affected
“Our group’s lesson plan, making ice cream in a bag, involved phases of matter and explored heat gain and loss required to change phases. The students were thrilled to be making ice cream, and they were all shocked at how delicious it was and that they could make it themselves. Solids, liquids, and gases were something that they learned in science class, so this expanded on that a little bit and was a concept they were already somewhat familiar with,” shares Dolce Whitwell ’23. “We all had a great time interacting with the kids! It was a nice change of pace, and it’s important for younger students to see college students in their environment, because it inspires them and helps them realize what they’re capable of.”
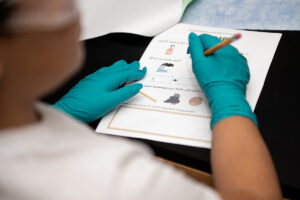
“The group I worked in had the general topic of the environment. We chose to make our lesson on acid rain because it’s something related to the environment, and it’s actually very common. We thought it would appeal to the children, as rain is something they have experienced, so they could apply what they learn into their daily lives. To show the kids how acid rain would react with different materials, we used vinegar as our rain because it is a weak acid,” says Sabrina Gonzalez ’23. “We got popsicle sticks (wood), chalk, pennies (copper), and rocks. After a brief introduction of what acid rain is and how it came to be, we had the kids each build a structure using all the materials. The kids made predictions on which material might get affected by the acid. Then, each kid poured ‘acid rain’ onto their structure using pipettes. They got to see how the chalk bubbled when reacting with the vinegar and see how reactions with acid rain can destroy many materials used for buildings and statues.”